Accelerated discovery of electrode-electrolyte interfaces under operating conditions
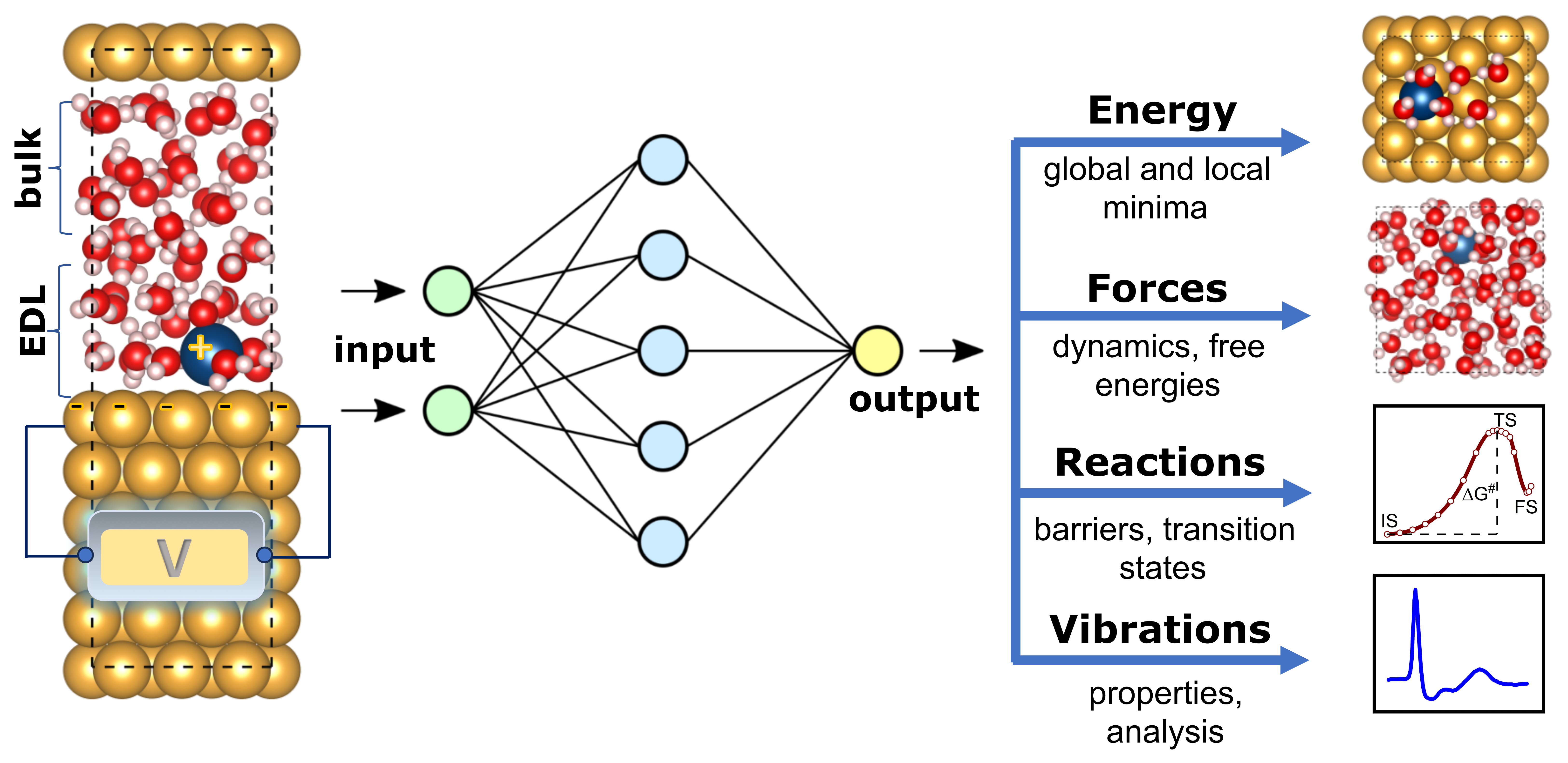
Comprehensive understanding of the processes at the electrode-electrolyte interfaces is crucial for the design of high-performance energy materials. However, modeling of electrocatalytic reactions remains a challenging endeavor due to several factors. First, electrochemical reactions occur in the region of electric double layer (EDL), therefore proper concentrations of ions and their impact on interfacial water rearrangement should be considered. Second, the critical parameter for electrochemical processes is the electrode potential. The commonly employed grand canonical ensemble method is constrained by the finite size of the simulation cell, and the change of electrode potential requires simultaneous adjustment of the number of electrons and electrolyte species, while a generally accepted grand canonical scheme is still missing. Third, and most important, computationally demanding, ab initio molecular dynamics (AIMD) simulations are needed to obtain clear atomistic description, which results in a pressing need to explore novel techniques. In the current age of rapid advancements in data science, the integration of machine learning (ML) and artificial intelligence (AI) with molecular dynamics shows promising potential to speed up simulations and analysis, while maintaining accuracy in predicting material properties. Our research will focus on the integration of modern theoretical approaches to facilitate the development of highly efficient electrode-electrolyte systems.